Authors: Marcy Belloy#, Benjamin A M Schmitt#, Florent H Marty , Charlotte Paut , Emilie Bassot , Amel Aïda , Marine Alis , Margot Zahm , Adeline Chaubet, Hugo Garnier , Thelma Flores-Aguilar , Elisa Roitg , Renzo Gutierrez-Loli , Sophie Allart , Romain Ecalard, Raphaël Boursereau , Gaëtan Ligat , Daniel Gonzalez-Dunia, Nicolas Blanchard , Elsa Suberbielle
When inflammatory processes occur inside the brain, this is referred to as neuroinflammation. For several years now, it has been established that neuroinflammation can disrupt brain function. Neuroinflammation is often associated with neurodegenerative diseases, but it can also be caused by infections, such as the SARS-CoV2 virus or the Toxoplasma gondii parasite. The T. gondii parasite, which persists inside neurons, causes chronic, low-level neuroinflammation and is responsible for various cognitive disorders. However, the nature of the inflammatory signals involved in these cognitive alterations and the mechanisms linking neuroinflammation and neuronal dysfunction remain poorly understood.
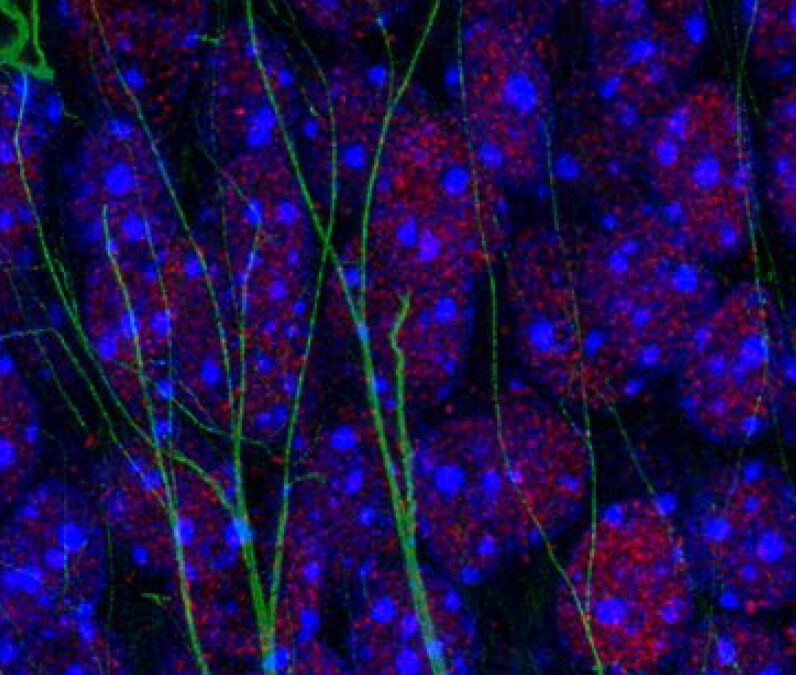
Recent discoveries have shown that spatial memory (which allows us, for example, to remember an experience in its context and find our way back) relies on a delicate balance between DNA breaks and repairs in neurons. This mechanism, known as epigenetic because it modifies the structure of DNA rather than its sequence, is essential for optimal neuron function, particularly in the region of the brain called the hippocampus, which is responsible for spatial memory.
A team of neuroscientists (led by Elsa Suberbielle) and immunologists (led by Nicolas Blanchard) from the Toulouse Institute for Infectious and Inflammatory Diseases (Infinity) have established a link between spatial memory deficits caused by T. gondii infection and an imbalance in the process of responding to DNA breaks in neurons. They identified an inflammatory signal (a cytokine called interleukin 1) involved in spatial memory deficits and highlighted the ability of this immune-derived signal to disrupt the epigenetic balance of certain neurons, thus providing the first molecular explanation for the memory disturbances caused by this chronic parasitic infection.
By manipulating the response to DNA breaks or the receptors of these inflammatory signals on neurons in experimental models, they were able to prevent memory disorders, even when caused by more severe inflammation of the brain.
This work, published in the journal Nature Neuroscience, has potential implications beyond T. gondii infection, as interleukin 1 is a signal that is commonly increased in many chronic inflammatory diseases. These results could suggest new therapeutic avenues for the treatment of memory deficits, for example in the context of depression or neurodegenerative diseases.
Link to the article: https://pubmed.ncbi.nlm.nih.gov/40841478/