Cell Imaging facility
The cellular imaging facility of INFINITy offers a wide array of biophotonic approaches, allowing to visualize biological events from the nanometric to the organ scale.
Microscopes are available for scientists from INFINITy but also from outside the institute (public or private domain).
Thanks to the connection of the facility with TRI (Toulouse Réseau Imagerie) and France BioImaging (FBI), the facility is ISO9001 V2015 and NFX 50-900 v2016 certified, in order to ensure a continuous improvement and customer satisfaction.
Our team
News
Trainings
The platform regularly organizes training courses for public and private partners.
- Inserm permanent training:
- 3D image analysis with Imaris software: March 3-5, 2026, in the computer room of the Inserm delegation in Toulouse. Speakers: Sophie Allart and Sébastien Marais (BIC, Bordeaux).
- QuPath training: From basics to advanced methods for analyzing large 2D images: June 10-12, 2026, at the Inserm delegation in Toulouse. Speakers: Remy Flores Flores (I2MC, Toulouse), Simon Lachambre, and Lhorane Lobjois
- CNRS Formation Entreprise:
- QuPath training: From basics to advanced methods for analyzing large 2D images: March 26-27, 2026, in Toulouse. Speakers: Simon Lachambre and Lhorane Lobjois
- 3D image analysis with Imaris software: April 28-30, 2026 in Bordeaux. Speakers: Sophie Allart and Sébastien Marais (BIC, Bordeaux)
- Doctoral school:
- QuPath training: From basics to advanced methods for analyzing large 2D images: March 16-17, 2026, in Toulouse. Speakers: Remy Flores Flores (I2MC, Toulouse), Simon Lachambre, and Brice Ronsin (CBI, Toulouse)
- Technology workshop:
- The basics of Python for microscopy image analysis. May 18-21, 2026, in Toulouse. Speakers: Erwan Grandgirard (IGBMC, Strasbourg), Lhorane Lobjois, Jerome Mutterer (IBMP, Strasbourg)
Registration on Sirène or by email to cindy.neves@inserm.fr
Services provided
For every new user, we organize a first meeting to help to choose the best protocol and to decide which microscope to use.
The facility offers two types of services:
- Training: A training provided by the engineers of the facility is mandatory to be granted an access to the microscopes and to book them
- Expertise: Engineers of the facility can help you for the technical development of your projects and for the analysis of your data.
Training, advice, competence
- Training to use microscopes
- Help for implementation or optimization of an experimental protocol
- Assistance for certain technical approaches
- Help for image analysis
- Expertise for private companies
- Continuing education (INSERM, CNRS, University, private company, Workshop)
Applications
Techniques available on the facility
- One-shot multiplex imaging
- Spectral imaging
- Time lapse
- FRET
- TIRF
- FRAP/ FLIP/ iFRAP
- IRM (Interference Reflection Microscopy)
- Biphoton
- STED
- Photoactivation
- uPAINT
Image analysis
- Object quantification
- Fluorescence intensity quantification
- Colocalization
- Macros for ImageJ
- 4D analysis with Imaris
- ImageJ
- QuPath
- Training for image analysis
Equipments
The technical imaging facility of Infinity offers tools to image your samples from the cellular to the tissular scale.
Confocal SP8-STED 3X microscope
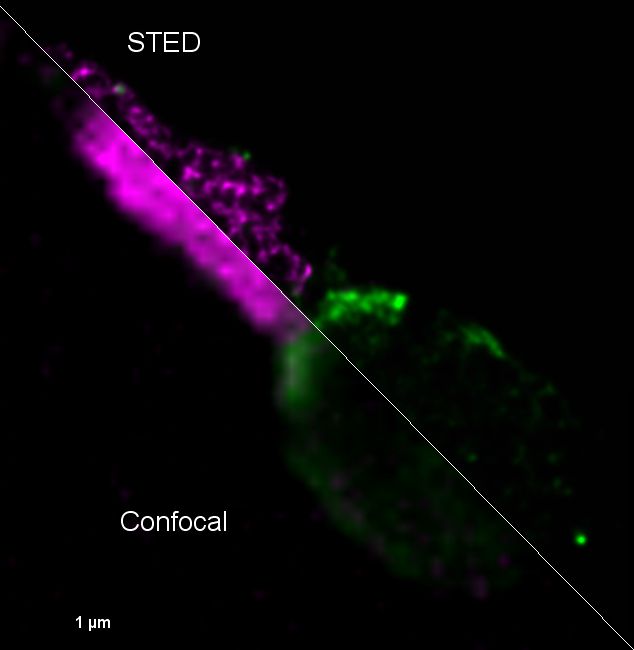
Mise en évidence du virus Zika dans les spermatozoïdes humains par microscopie de super-résolution STED. Tom20-StarRed (Mitochondries en vert) et 4G2-Alexa-594 (Virus Zika en violet). Elsa Suberbielle, équipe Dunia, CPTP.
Lasers: 405 nm, 488 nm, 532 nm, 552 nm and 635 nm on an inverted motorized microscope
- For STED acquisition, excitation lasers are 532 and 635 nm. Depletion laser is 775 nm.
- Objectives: 20 X Plan Fluotar DIC (NA: 0.8), 63x Oil plan apo DIC (NA 1.4) and a 100X (NA 1.4) for STED acquisitions
- The system is equipped with 3 high sensitive HyD (QE: 45 % instead of 25 % for PMT) and 2 PMT.
- Equipped with temperature and oxygen controllers.
Confocal LSM 710 microscope
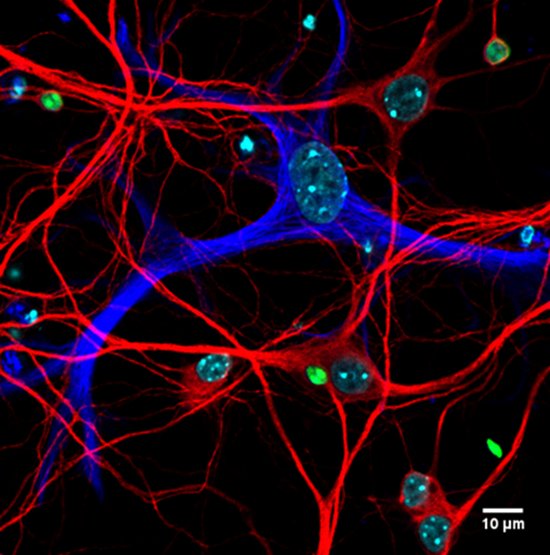
A mouse hippocampal neuronal culture infected by Toxoplasma gondii tachyzoïtes (Red: MAP2, Blue: GFAP, Green: T. gondii), Marcy Belloy, Equipe Blanchard
- Lasers: 405 nm, 458 nm, 488 nm, 514 nm, 561 nm et 633 nm on an inverted motorized microscope
- Objectives: 20x plan APO (NA: 0.8), 40x oil plan APO (NA:1.3) and 63x oil plan APO (NA 1.4).
- Equipped with temperature and oxygen controllers.
- With the spectral detector, autofluorescence can be removed and dyes which are spectrally close can be separated.
- FRAP and colocalization modules are available.
MICA Microscope
 Automated, fully motorized inverted wide-field microscope for fluorescence and histochemistry. Suitable for 2D and 3D mosaic acquisitions.
Automated, fully motorized inverted wide-field microscope for fluorescence and histochemistry. Suitable for 2D and 3D mosaic acquisitions.
4 diodes (365nm, 470nm, 555nm, 625nm) for simultaneous acquisition of 4 fluorochromes using 4 sCMOS sensors.
Objectives: 1.5X, 10X, 20X, 63X auto water immersion
On-the-fly deconvolution in 2D and 3D.
Inserts: 1 slide, 4 slides, plates.
Confocal Spinning disk / TIRF/ FRAP microscope
- Lasers: 375 nm (for photoactivation), 405 nm, 491 nm, 561 nm and 642 nm.
- Objectives: 10X Plan Apo (NA 0.75), 20X plan apo (NA 0.75), 40X oil immersion fluor (NA 1.3) suited for UV experiments, 60X oil immersion plan apo (NA 1.4) and 100X oil immersion apochromate (NA 1.49) for TIRF experiments. DIC can be acquired with all the objectives.
- The CSU-X1 confocal scanner unit allows ultra-fast acquisition times, while limiting photobleaching.
- ILas² module allows FRAP/FLIP/photoactivation experiments and also azimuthal TIRF and dSTORM.
- The microscope is fully encaged and regulated for temperature and CO2 to maintain cells in optimal growth conditions.
Microscope Apotome 2
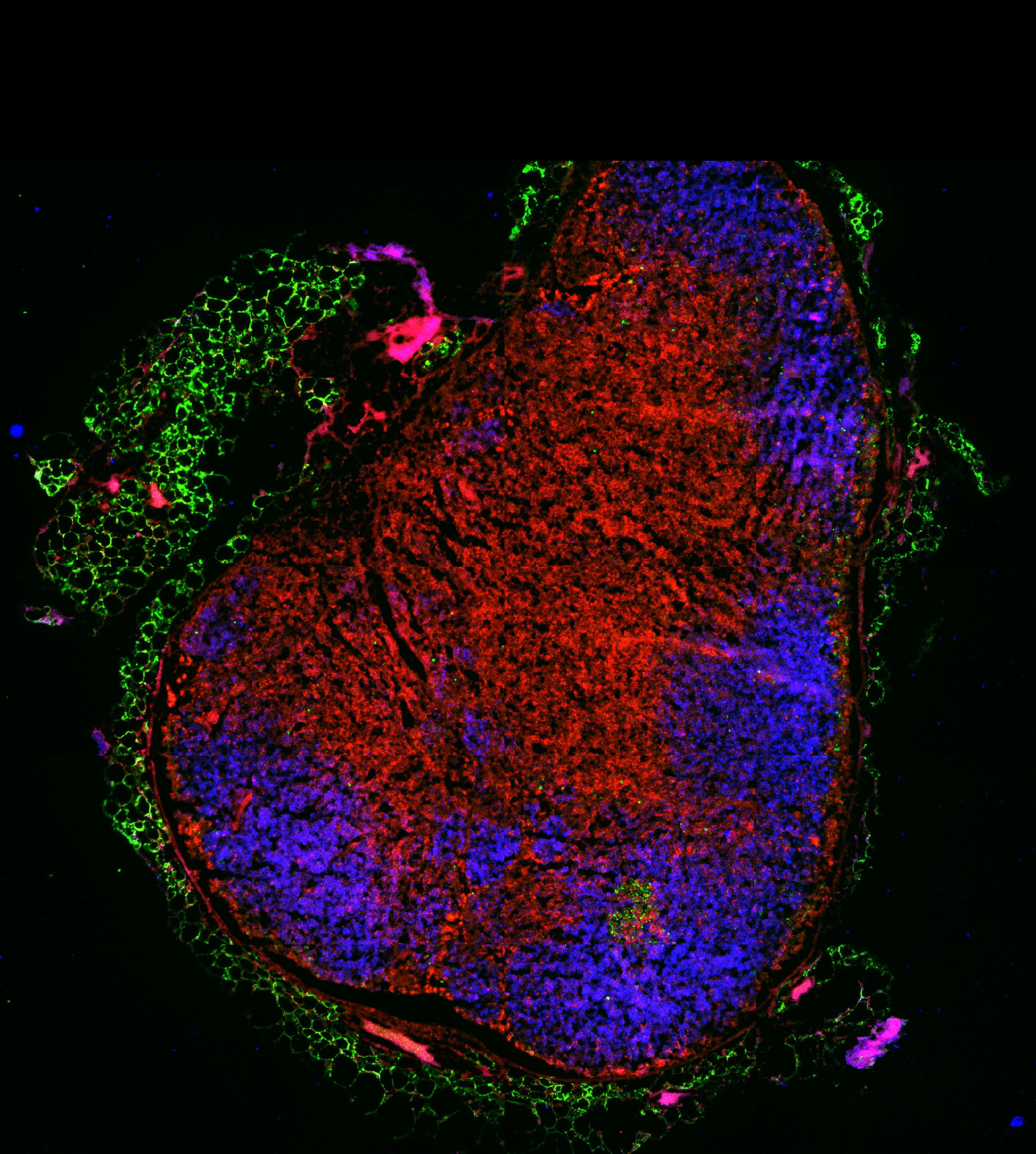
Meryem Aloulou, Team Nicolas Fazilleau
- This microscope can be used as a wide field but it can also remove out of focus fluorescence to allow optical sectioning.
- It is also possible to perform image tiling with a perfect stitching.
- Exact positions can be studied for several days with a perfect repositioning.
- The microscope is fully encaged and regulated for temperature and CO2 to maintain cells in optimal growth conditions.
- Objectives: 10X, 20X, 40X and 63X.
- Fluorescent filters for DAPI, Green, Red and Far Red.
Upright 2 Photon BRUKER 2P PLUS
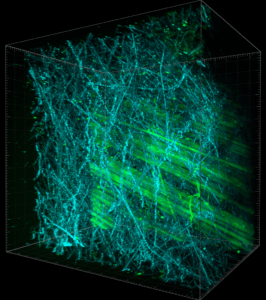
UBI-GFP mouse skin. Collagen fiber in SHG (Blue) and fibroblast in GFP (Green). Lhorane Lobjois and Claire Murat
- Upright multi photon Bruker 2P Plus microscope for cellular and tissular multicolor acquisitions thanks to 4-channel NDD. The pulsed laser Chameleon Discovery is adjustable from 680 to 1340 nm.
- Objective: dipping 20X Plan-Apo (NA: 1), Olympus.
- The microscope is equipped with a Galvo scanner for classical acquisitions and a resonant scanner for rapid live acquisitions. A 400µm piezo electric Z motor enables rapid acquisition of tissue thickness.
- It is also equipped for tissue explant for hours (oxygenation and thermoregulation), and for microscopy in small animals.
Upright 2 Photon microscope ZEISS LSM 7MP
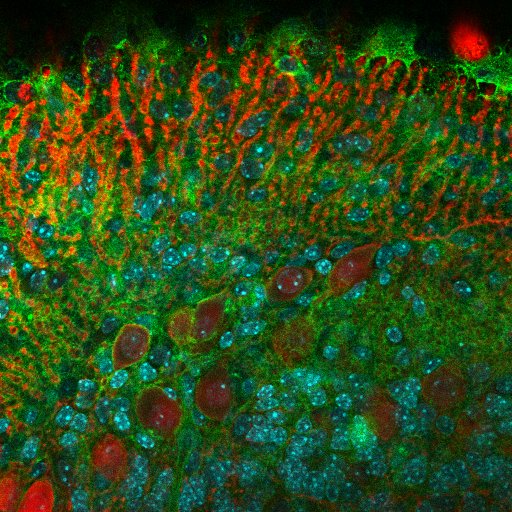
Purkinje cells are labelled with calbidin (red), nucleus with Dapi (cyan) and CMH class 1 in green – Lidia Yshii, Team Roland Liblau
- Upright multi photon Zeiss LSM 7MP microscope for cellular and tissular multicolor acquisitions thanks to the 5-channel NDD. The pulsed laser Chameleon Ultra II is adjustable from 690 to 1080 nm.
- Objectives: dipping 20X Plan-Apo (NA: 1), 40X oil Plan-Apo (NA: 1.4) and 40X water immersion (NA: 1.1).
- The microscope is fully encaged and regulated for temperature and CO2 to maintain cells in optimal growth conditions.
- It is also equipped for tissue explant for hours.
Image analysis
The imaging platform provides two workstations for image processing and analysis. They are also used to deconvolute wide-field images using distributed computing techniques. Upon request, the platform’s engineers write automation sequences in ImageJ or QuPath (macros) to automate image analysis. Training sessions are regularly organized to help users learn how to use the main imaging software programs.
The software available are:
- QuPath: Free software for analyzing large 2D images. Segmentation and classification with integrated artificial intelligence modules. Automation via scripts
- ImageJ: Free software for analyzing 2D and 3D images. It allows the creation of task automation for repetitive processes.
- Imaris: Software for processing and analyzing stacks of 3D images. It allows surface modeling, spot detection and tracking over time, reconstruction of cell extensions (e.g., dendrites and spines), all associated with statistical data. It also allows the creation of animations for presentations and supports the main file formats used in microscopy.
- Zen Light and LSM Browser (Zeiss): for viewing images acquired with Zeiss microscopes (.lsm and .czi files).
- LAS X Leica Lite: for viewing images acquired with the Leica SP8 microscope (.lif files).
Other information
Application forms
Forms to fill for new users:
Steering comittee
- Dr L. Dupré (Infinity-Eq Lesourne/Dupré) – Scientific Officer
- Dr D. Dunia (Infinity-Eq Dunia/Malnou) – Scientific Officer
- Dr S. Guerder (Infinity- Eq Fazilleau/Guerder)
- Dr N. Blanchard (Infinity-Eq Blanchard)
- Dr M. Savignac (Infinity-Eq. Guery)
- Dr M. Diaz-Munoz (Infinity-Eq Diaz-Munoz)
- Dr F. Briand –Messange (Infinity-Eq. Poupot/Ausseil)
- Dr M. Requena (Infinity-Eq Izopet)
- Dr N. Gaudenzio (Infinity-Eq Gaudenzio)
- Dr N. Jabrane-Ferrat (Infinity-Eq Jabrane-Ferrat)
- Dr A. Astier (Infinity-Eq Liblau/Saoudi)
- Dr J. Kamphuis (Infinity-Eq Reber)
- Dr O. Joffre (Infinity-Eq van Meerwijk/Joffre)
- Dr C. Leprince (Infinity-Eq Simon)
- Dr C. Bonnard (IRSD)
Publications
2026 |
Investigating Dysregulated X Chromosome Inactivation in Human B Cells and Plasmacytoid Dendritic Cells by RNA FISH Journal Article In: Methods Mol Biol, vol. 2990, pp. 157–177, 2026, ISSN: 1940-6029. |
2022 |
Identification of bacterial lipopeptides as key players in IBS Journal Article In: Gut, 2022. |
Ca(v)1.4 calcium channels control cytokine production by human peripheral T(H)17 cells and psoriatic skin-infiltrating T cells Journal Article In: J Allergy Clin Immunol, vol. 149, no. 4, pp. 1348-1357, 2022, ISSN: 1097-6825 (Electronic) 0091-6749 (Linking). |
2020 |
Glycerophosphodiesterase 3 (GDE3) is a lysophosphatidylinositol-specific ectophospholipase C acting as an endocannabinoid signaling switch Journal Article In: Journal of Biological Chemistry, vol. 295, no. 46, pp. 15767–15781, 2020, ISSN: 1083351X. |
2019 |
The Actin-Based Motor Myosin Vb Is Crucial to Maintain Epidermal Barrier Integrity. Journal Article In: The Journal of investigative dermatology, vol. 139, no. 7, pp. 1430–1438, 2019, ISSN: 1523-1747 (Electronic). |
2018 |
In: J Allergy Clin Immunol, vol. 142, no. 3, pp. 892-903, 2018, ISSN: 1097-6825 (Electronic) 0091-6749 (Linking). |
TLR7 escapes X chromosome inactivation in immune cells Journal Article In: Sci Immunol, vol. 3, no. 19, 2018, ISSN: 2470-9468 (Electronic) 2470-9468 (Linking), (In the top 5% of all research outputs scored by Altmetric. http://www.altmetric.com/details/32261033). |
Switching between individual and collective motility in B lymphocytes is controlled by cell-matrix adhesion and inter-cellular interactions Journal Article In: Scientific Reports, vol. 8, no. 1, pp. 5800, 2018, ISSN: 2045-2322. |
The Wiskott-Aldrich Syndrome Protein Contributes to the Assembly of the LFA-1 Nanocluster Belt at the Lytic Synapse Journal Article In: Cell Rep, vol. 22, no. 4, pp. 979-991, 2018, ISSN: 2211-1247 (Electronic). |
Vectorial Release of Hepatitis E Virus in Polarized Human Hepatocytes Journal Article In: J Virol, 2018, ISSN: 1098-5514 (Electronic) 0022-538X (Linking). |
2016 |
CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice Journal Article In: Proc Natl Acad Sci U S A, vol. 113, no. 39, pp. 10956-61, 2016, ISSN: 1091-6490 (Electronic) 0027-8424 (Linking). |
2015 |
Intravacuolar Membranes Regulate CD8 T Cell Recognition of Membrane-Bound Toxoplasma gondii Protective Antigen Journal Article In: Cell Rep, 2015, (Nov 23. pii: S2211-1247(15)01288-7. doi: 10.1016/j.celrep.2015.11.001.). |
Alumni
- Josselin Ragu, Stagiaire M2 (2025)
- Lucie Demeersseman, CDD Inserm (2021)
- Danielle Daviaud, IE Inserm (2017-2020)
- Astrid Canivet, IE Inserm (2009-2020)
- Raïssa Houmadi, PhD (2012-2017)
- Magda Rodrigues, IE Inserm (2008-2016)
- Daniel Sapede, CDD Inserm (2009-2010)
- Sabina Mueller, IE Inserm (2001-2005)
Contact & booking
Equipments of the facility are in self-service access after a mandatory training. Booking can be done on the TRI website (demandée).
The imaging facility of Infinity is located at the second floor of the F building, Pavillon Lefebvre, within the CHU Purpan.
Member of TRI-GENOTOUL (Toulouse Réseau Imagerie) and France BioImaging (FBI) – ISO 9001:2008 since january 2010 and NF X50-900 since february 2014.

Member of RTMFM – Microscopie Photonique de Fluorescence Multidimensionnelle.

Member of GDR CNR–ImaBio






